Abstract
Introduction
AMG-176 belongs to a class of small molecule inhibitors known as BH3 mimetics, which mimic the role of BH3-only proteins and inhibit pro-survival Bcl-2 family proteins. AMG-176 specifically inhibits the pro-survival Mcl-1 protein. Overexpression of anti-apoptotic protein Mcl-1 has been extensively reported in hematological malignancies including mantle cell lymphomas (MCL), diffuse large B cell lymphomas (DLBCL), and multiple myeloma (MM). Chromosomal aberration of Mcl1 was identified as one of the most important survival genes for MM, follicular lymphoma (FL), MCL, and DLBCL, and is associated with shorter patient survival. These hematological malignancies are targetable by FDA-approved chimeric antigen receptor (CAR) T cell therapies with varying efficacies as single agent regimens. The role of Mcl-1 in T cell biology could be further elucidated to improve therapeutic outcomes of malignancies characterized by Mcl-1 overexpression. Here, our team explores the effect of Mcl-1 inhibitor, AMG-176, on T cell quality and function.
Methods
Our lab obtained healthy human donor blood from consenting adults, then isolated pan T cells using microbead negative selection (Miltenyi Biotec). Following isolation, T cells were cryopreserved in cryopreservative medium consisting of 90% fetal bovine serum (Corning) and 10% dimethyl sulfoxide (Sigma-Aldrich). Upon thaw, T cells were cultured in complete RPMI supplemented with [10ng/mL] h-IL-7 (Peprotech) and [100IU/mL] h-IL-2, and activated for 24 hours using αCD3/αCD28 coated beads (Thermofisher). At 24 hours post-activation, cells were exposed to various concentrations of AMG-176 (MedChem Express). Following 72-hour exposure, T cells were harvested and assessed for ATP production, viability, proliferation, immunophenotype, and effector function. To determine ATP production, we used a commercially available luminescence-based assay (Promega) and obtained our readout using a BioTek Synergy HTX plate reader, T cell immunophenotype was identified following 72-hour exposure to AMG-176 at 0nM, 10nM, 100nM, 500nM, and 1uM concentrations. Samples from each cohort were stained with Live/dead yellow viability dye, CD3, CD4, CD8, CD69, CD25, and CD127, then acquired using a BD LSRII flow cytometer. Flow cytometry data was analyzed using FCS Express analytical software (De Novo Software). Intracellular cytokine staining was performed using markers for Granzyme B, Perforin, and IFN-γ.
Results
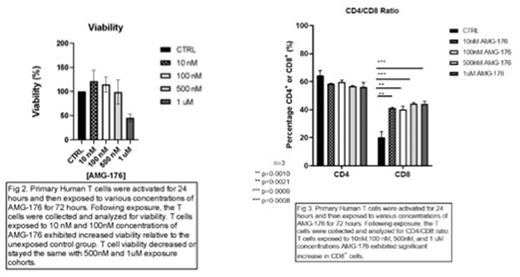
T cells exposed in vitro to 10nM and 100nM concentrations of AMG-176 exhibited greater ATP production (Fig 1.), viability, and proliferation (Fig. 2) than the unexposed control group. T cells exposed to 500nM or 1uM concentrations of AMG-176 exhibited unchanged or decreased ATP production, viability, and proliferation relative to the unexposed control group. T cells exposed to AMG-176 significantly increased CD8+ T cell frequency at 10nM and 100nM concentrations (Fig. 3), without affecting viability of CD3+ Pan T cells. Our flow data also indicates dose-dependent increase in expression of early activation marker CD69+.
Conclusion
Exposure of human T cells to Mcl-1 inhibitor, AMG-176, provides an increase in ATP production, viability, proliferation, and activation. These results shed light on the role of Mcl-1 in T cell biology. Furthermore, increases in human T cell viability, proliferation, and function as facilitated by AMG-176 could offer insight into the advancement of effector cell-based therapies, such as chimeric antigen receptor (CAR) therapy. We may employ AMG-176 alongside CAR T therapy in order to achieve more effective clinical outcomes.
Disclosures
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal